Complete the Statements About the Redox Reaction Below.
Complete the statements about the redox reaction below. In the reaction MgCl2MgCl2 the correct half-reaction for the oxidation that occurs is A.

Half Reaction Easy Science Redox Reactions 10th Grade Science Easy Science
B O2 is the substance oxidized.

. 2Au aq 3Cd s 2Au s 3Cd2 aq Half-reactions. Br2 Br- I-. Of O in H2O.
Write down the unbalanced equation skeleton equation of the chemical. Cu2 aq Cu aq E015 V Fe2 aq 2e Fe s E044 V 3 4 is being. For the redox reaction given below complete the following.
B A reaction can result in either oxidation or reduction not both. B What is the oxidation no. Cr2O3 Mg ----- MgO Cr a What is the oxidation no.
Mc029-1jpg Which statement correctly describes a half-reaction that is taking place. Electrolytic is being reduced. 2H s Sn s Sn2 aq H2g a Sn2eSn2 b SnSn22e c 2HH22e d.
The reaction is slow. Of Al in Al203 c Find the. Which of the following statements correctly describe a redox reaction.
4AI s 302 g 2AI2O3 s a What is the oxidation no. Of Fe in FeCl2. The reaction in which hydrogen is gained or oxygen is lost by a substance is called reduction reaction.
B What is the oxidation no. Each of these half-reactions is balanced separately and then combined to give the balanced redox equation. 20 Complete the statements about the redox reaction below.
Solution for Complete and balance the following redox reaction in an acid solution. A A reaction involving elemental oxygen is a redox reaction. For a particular redox reaction NO is oxidized to NO3.
Cd aq is acting as an reducing agent. Complete the following statements that refer to the reductionoxidation or redox reaction which occurs when Zns metal is put into an aqueous solution with Cu 2 aq ions. Zns Cu 2.
Au aq is acting as an oxidizing agent. Break the reaction into the two half reactions. Of Cr in Cr2O3.
Of O in O2. That is when H2Og is introduced into a. Using the half- reaction method balance the redox reaction shown below.
Solve any question of Redox Reactions with- Patterns of problems. B What is the oxidation no. For the redox reaction given below complete the following.
Ozg 2H2Ol 4e - 40H aq E 040 V. Of S in SO2 Choose. 1 points O2g 2H2O1 4Ce3aq 40H aq 4Ceaq Half-reactions.
I-Br2 --- IO3-Br- Step 1. For the redox reaction given below complete the following. Below is a metabolic redox reaction that occurs within mitochondria.
Consider the redox reaction below. Analyze the reaction to complete the following paragraph. In the electrochemical cell using the redox reaction below the anode half-reaction is _____.
Choices may be used more than once. The reaction will not occur. Select all that apply-Multiple select question-Electrons always move from an atom that attracts them less strongly.
FeCl2 H2O2 H FeCl3 H2O a What is the oxidation no. 2Cu2 a Fe s- 2Cu aq Fe2 aq Half-reactions. In the redox reaction 4Fe 3 O2 12HCl 4FeCl3 6H2O A HCl is the reducing agent.
C Fe undergoes an oxidation number decrease of 3 units per atom. Mg Mg2 2e D. B What is the oxidation no.
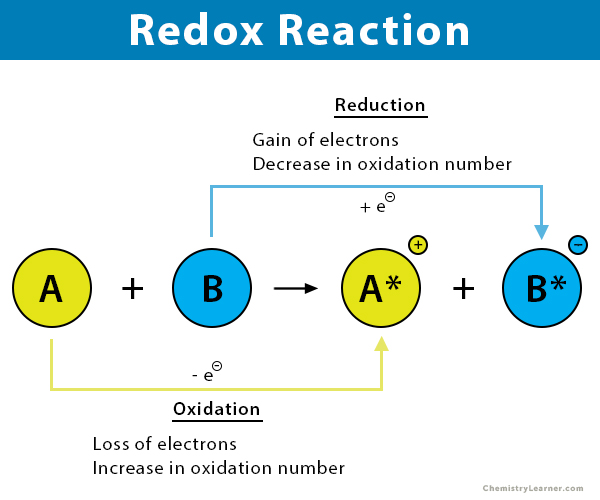
Complete the statements about the redox reaction below. D Oxidation is the loss. C Reduction is the gain of electrons.
Of Cl in ClO3. Cl2 2e 2Cl C. Complete and balance the following redox reaction in.
For the redox reaction given below complete the following. SO2 SO4² Cr a What is the oxidation no. Start your trial now.
First week only 499. Hydrogen is oxidized from 1 to 0. Au aq 3e - Au s E 150 V Cd2 aq 2e - Cd s E -040 V is being oxidized.

Mno 4 Aq Al S Mn2 Aq Al3 Aq In 2022 Redox Reactions Reactions Solutions
No comments for "Complete the Statements About the Redox Reaction Below."
Post a Comment